This paper entitiled “Effect of Aliphatic Chain Length on the Stress–Strain Response of Semiaromatic Polyamide Crystals” a follow-up study of our first paper on high-performance polyamides in collaboration with ExxonMobil.
A lot of effort was put into this work, thanks to the great support from Professor Martini and ExxonMobil (especially the coauthors of this paper: Wenjun Li, Spencer T. Stober, Adam B. Burns, and Manesh Gopinadhan), this paper finally was published today after two rounds of reviewer’s response and tremendous corrections and addtional supporting materials. From the first submission on January 17th in 2022 to the final publication on June 8th in 2022, it lasted almost 5 months.
Abstract:
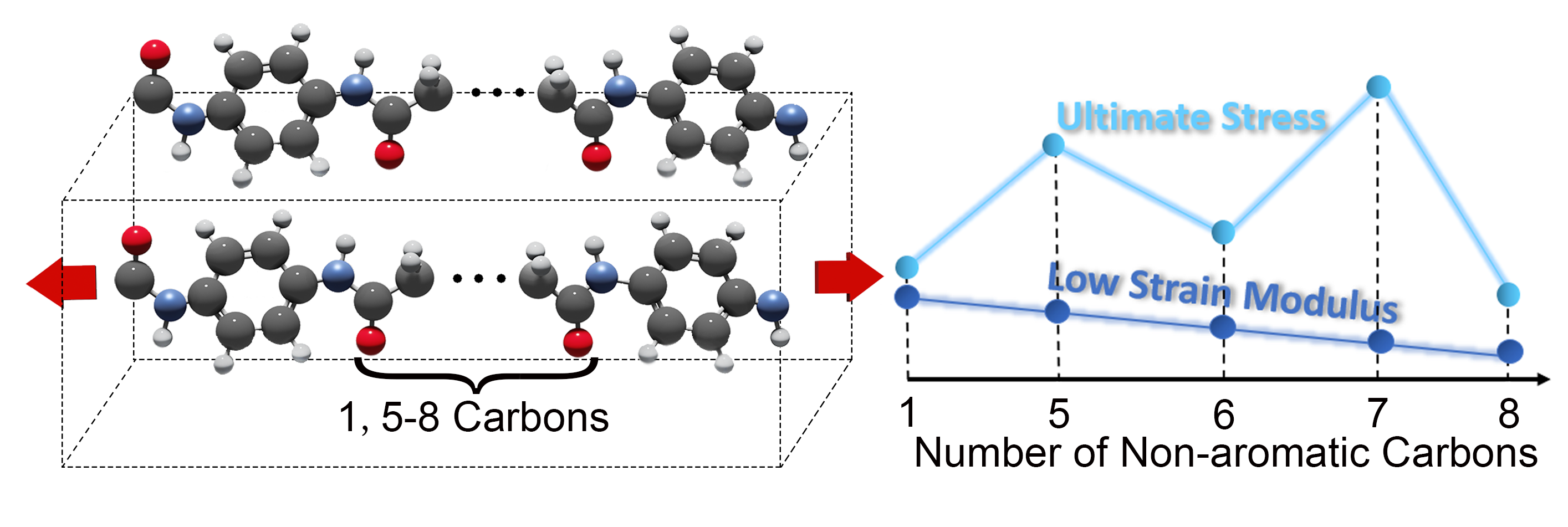
Reactive molecular dynamics simulations were used to model poly(p-phenylene terephthalamide) and related aromatic–aliphatic polyamides derived from p-phenylenediamine and aliphatic diacids with different numbers of carbon atoms in the aliphatic chain (5, 6, 7, or 8). Tensile strain was applied to each polymer crystal in the chain direction, and the mechanical response was characterized. All the polymers with aliphatic segments exhibited strain hardening, transitioning from an initial (low-strain) linear regime to a second (high-strain) linear regime. The modulus at high strain was similar for all polymers, but the modulus calculated at low strain decreased with increasing aliphatic chain length. The decrease in the low-strain modulus with increasing chain length was explained by the observation that polymers with longer aliphatic chains were wavier (i.e., deviated more from the fully extended conformation) in the quiescent state such that they could accommodate low strain without deforming covalent bonds. Extension of wavy chains occurred through an intrachain process for all polymers, quantified by the bond dihedral angles. In addition, for polymers with an even number of non-aromatic carbons, the strain response involved slip between chains within the hydrogen-bonded sheets. The ultimate stress of the polymers exhibited an odd–even effect (even polymers were weaker) which was explained by differences in hydrogen bonding and ring–ring coplanarity prior to failure; polymers with an even number of carbon atoms had less favorable H-bonding and poorer ring alignment. The results revealed direct correlations between aliphatic chain length, intra- and interchain interactions, and the mechanical properties of polyamide crystals.